MOLINE, Ill. — It's a hot and humid summer day in mid-June. Nothing too surprising given the time of year is near the summer solstice, the point at which the maximum amount of solar energy is reaching North America. You decide to jump into a local lake, surprised that the water remains chilly. Water temperatures are in the middle 50s. Why is that the case? Why does water take much longer than air to heat up? Let's dig in!
Specific heat
What is specific heat? It is the heat energy required to raise the temperature of a given amount of substance by one degree Celsius. Water and air have very different specific heat values. For example, water has a specific heat of 4.186 J/g while air has a specific heat of 1.005 J/g. The measurement unit is Joules, which is the amount of energy, and grams, the amount of substance.

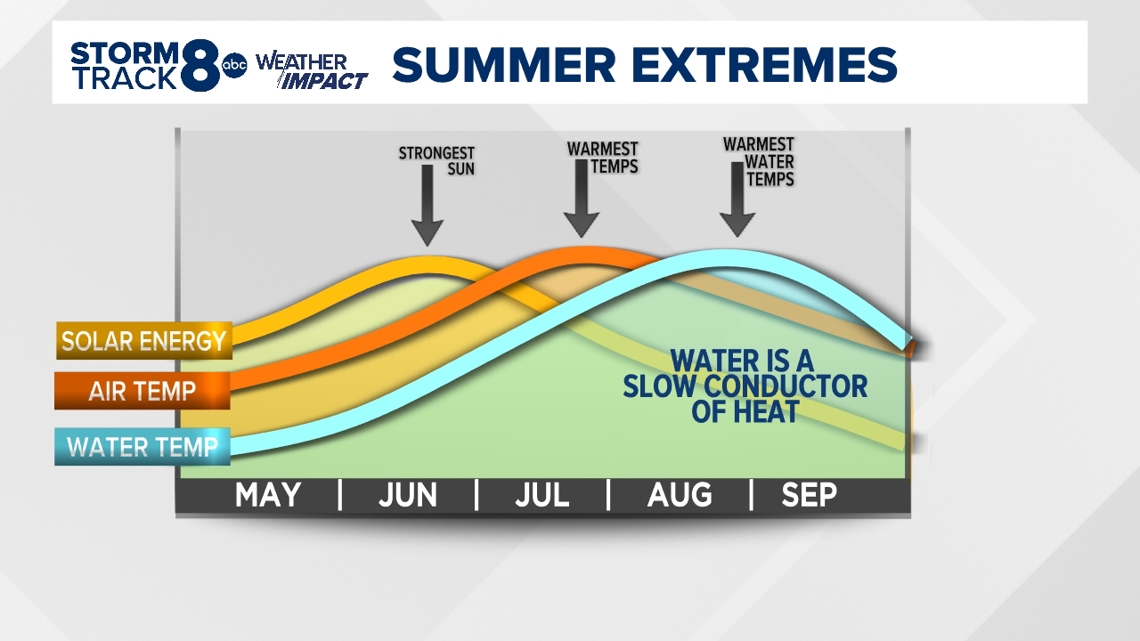
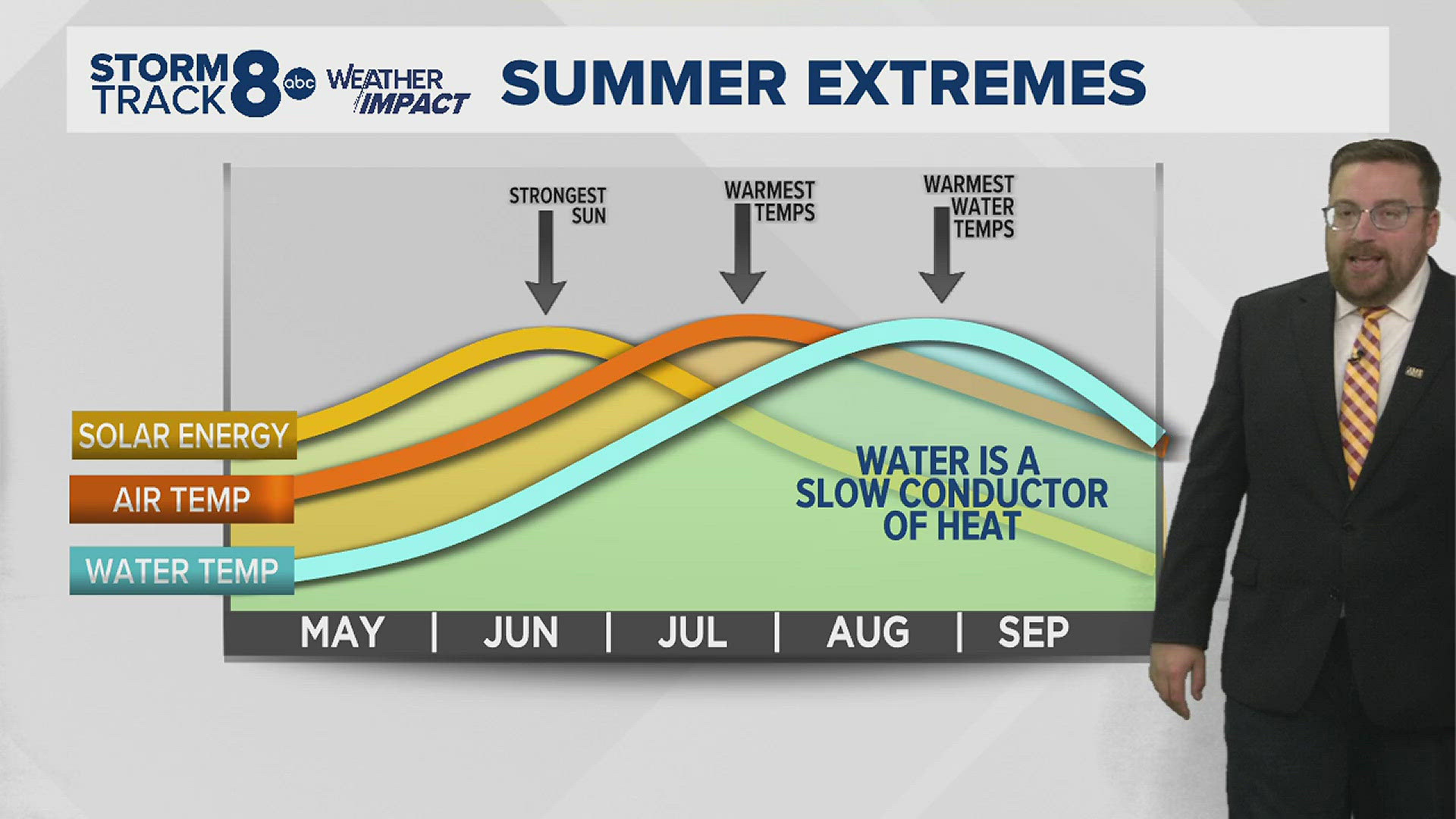
A delayed reaction
In the graph above notice how the warmest water temperatures often occur well after the warmest air temperatures of the year and the strongest solar energy, too. Water has a much higher specific heat capacity, meaning water takes longer to heat up and cool down. This is why large bodies of water impact our weather patterns not just in the Midwest, but around the world.
Looking at temperature trends for a coastal city or town, you'll notice very little change over short periods. Being next to a large body of water often stabilizes temperature patterns, whereas here in the Midwest, our temperature patterns can vary greatly from day to day.
So, if you are looking for a more comfortable water temperature for swimming, you'll likely get it later in July and especially in August.
Have a question that you would like me to answer for an upcoming Ask Andrew segment? Submit it, here!