Salt is our savior during big winter weather events but have you ever wondered how it works?
You probably learned in second grade that water freezes at 32 degrees Fahrenheit. If road surface temperatures are below 32 degrees, that means anything that falls onto it will freeze on contact. So when it rains, we get ice and when it snows, we get accumulation.
Road crews spread salt on the snow to lower the freezing temperature of the pavement. Typically, the salt that is put down onto roads works down to about 20 degrees. When it's colder than that, plows and sand are the only things that work.
During times of rainfall with temperatures below freezing, much of the salt that is applied washes off the roads which makes freezing rain situations even more dangerous for travel.


Another thing to think about is ice cream! Did you know that salt is essential to making ice cream? According to HowStuffWorks, salt mixed with ice creates a salt-brine that has a temperature lower than 32 degrees. That brine on the outside of the ice cream maker causes the cream to drop below freezing. Normal ice water is not cold enough to freeze the ice cream.

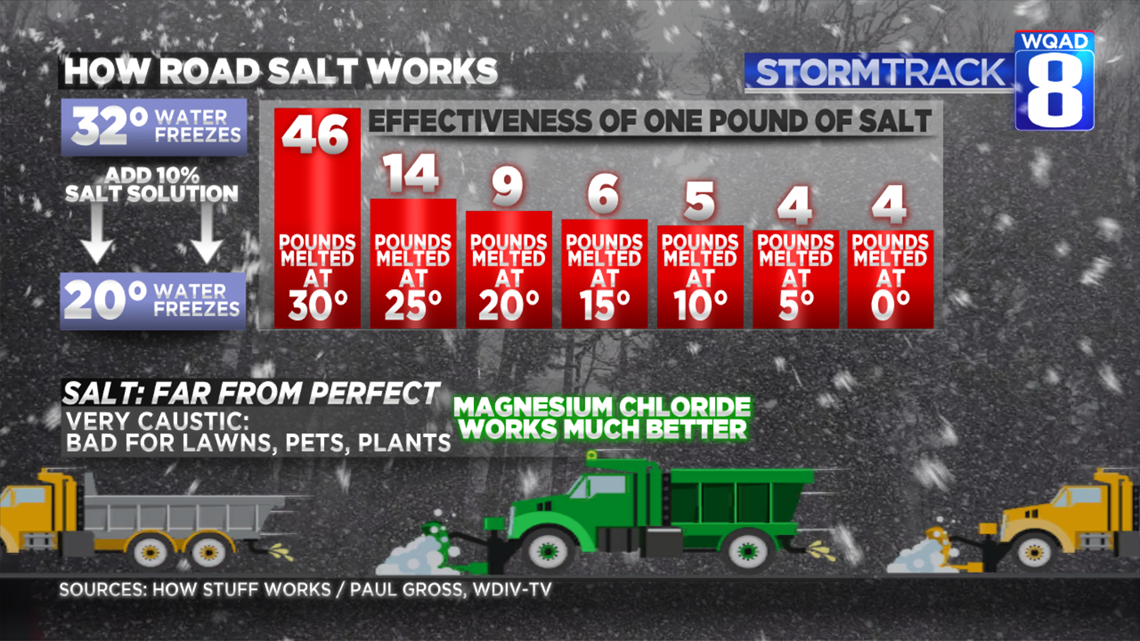
One thing to remember: while salt is highly effective between 30 and 32 degrees, its effectiveness goes down drastically with temperatures below 30. One Illinois Department of Transportation plow driver I talked to said that during Sunday's blizzard, he was putting down more than 500 pounds of salt per mile on Interstate 88. All in an effort to keep travelers safe.
-Meteorologist Eric Sorensen



